KJ3055-Chapter
8 (x-Ray Spectrometry)
Bremsstrahlung (Braking radiation)
|
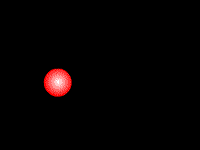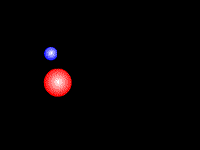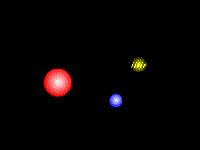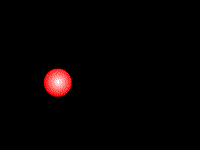
Fig. 1 |
††††††††††† Bremsstrahlung (from German bremsen (to
brake) and Strahlung (radiation)) (Fig.
1) is X ray radiation (yellow)
emitted by charged particles, such as electrons (blue),
which are braking around other charged particles, such as an atom nucleus (red). It forms the continuum component
of the x-ray spectrum generated by an x-ray
tube.
††††††††††† The spectral distribution of the braking
radiation is given by Kramer formula
(1)†††††††††††††††††††††††††††††††††††††††††††††††† 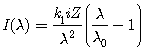
where I is radiation intensity, k1 is an empirical constant, i is the tube current, Z is the atomic number of the target
element, ![]() is the wavelength and
is the wavelength and ![]() is the cut off wavelength (i.e. the wavelength at which I = 0).
is the cut off wavelength (i.e. the wavelength at which I = 0).
††††††††††† Electron energy is eU, where e is the electron charge and U
is the tube voltage[i]. If U is in kV and e = 1, energy value in keV is:
(2)†††††††††††††††††††††††††††††††††††††††††††††††††††††††† ![]()
When an
electron hits the target, it slows down to a lower velocity and its kinetic
energy decreases as:
(3)†††††††††††††††††††††††††††††††††††††††††††††† ![]()
Here, m and
v are electron mass and velocity, respectively.
††††††††††† The energy lost by the colliding
electron turns into radiant energy associated to a photon:
(4)†††††††††††††††††††††††††††††††††††††††††††††††††† ![]()
Where h is the Planck constant, c is light velocity in vacuum, ![]() †is the frequency and
†is the frequency and ![]() †the wavelength of the associated wave.
†the wavelength of the associated wave.
††††††††††† The most energetic photon results
when the electron brakes to zero velocity in one single step and its total
energy is transferred to a photon, i.e.![]() . In this case we have:
. In this case we have:
(5)††††††††††††††††††††††††††††††††††††††††††††††††† ![]()
Therefore, the
wavelength of the resulting photon assumes the cut off value (![]() ) according to Duane-Hunt equation:
) according to Duane-Hunt equation:
(6)††††††††††††††††††††††††††††††††††††††††††††††††††††††††† ![]()
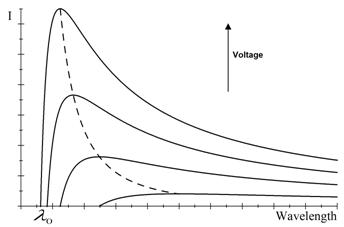
For a specific
U, no radiation with a shorter
wavelength is emitted (Fig.
2). Using numerical values for h and c, and with ![]() †in nm, it results:
†in nm, it results:
(7)††††††††††††††††††††††††††††††††††††††††††††††††††††††† ![]()
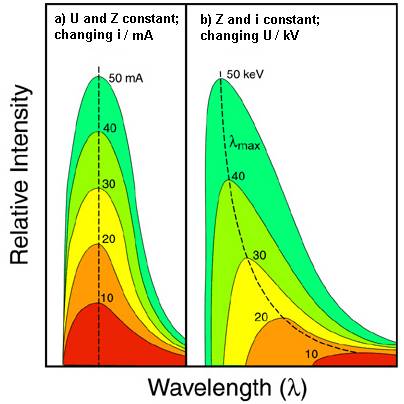
††††††††††† According to Kramer formula, at a
given ![]() radiation intensity increases proportionally with i (Fig. 3 a) and Z.
radiation intensity increases proportionally with i (Fig. 3 a) and Z.
††††††††††† An increase in U also bring about a rise in intensity, but, at the same time, the
cut off wavelength shifts to lower values (Equation ) and the maximum on the curve also
sifts in the same direction (Fig.
2 and 3b). The maximum intensity is given by Ulrey
formula:
(8)†††††††††††††††††††††††††††††††††††††††††††††††††††††† ![]()
where k2 is an empirical constant.
††††††††††† Kramerís formula is an approximation of the spectral
distribution. Its derivation ignores the self-absorption of x-rays and electron
backscattering effects.
|
Fig. 3. Effect of changing X-ray tube current (a), and accelerating
potential (b) on the continuous spectrum. Source |
In plasma
the free electrons are constantly producing Bremsstrahlung in collisions with
the positive ions. This contributes to the background signal in plasma emission spectrometry (Chapter
6).
Literature
1. H. Ebel,
X-Ray Spectrometry 1999, 28, 255.
2. E. Haug, W. Nakel , The elementary process of
Bremsstrahlung, World Scientific, River Edge, 2004. ISBN
9812385789
3. B.
Beckhoff,
___________________
F. G. Banica, 09-0-25